Abstract
Introduction: Cellular therapies promise new treatment options and potentially curative efficacy for a wide array of acute and chronic diseases. The ability to obtain viable, uncontaminated lymphocyte concentrates from peripheral blood is in high demand. A major barrier for translating lymphocyte-based therapies into clinical practice is the poor scalability of manual, labor- and time-consuming 'open system' methods (e.g. Ficoll density gradient centrifugation) currently being used for isolating lymphocytes. To address the well-known limitations of existing separation methods, we developed a two-stage cell isolation approach that enables highly-efficient extraction of lymphocytes from whole blood (WB) without the use of centrifugation or any open-air manipulations by (1) depleting ~99% of red blood cells (RBCs) from the sample via rapid sedimentation induced by HES (6% hydroxyethyl starch in 0.9% sodium chloride), and then (2) passing the leukocyte-rich supernatant through a high-throughput microfluidic device (based on our newly-developed approach of 'controlled incremental filtration,' CIF) to separate lymphocytes from platelets and residual RBCs.
Materials and Methods: WB was collected from healthy consenting volunteers under an IRB approved protocol. An optimization study was performed to identify parameters of the sedimentation protocol with the highest lymphocyte yield and minimal contamination. This optimal protocol was then used to compare performance of (i) the standard Ficoll density gradient separation method, and (ii) our new two-stage separation approach. Each WB sample was divided into two equal parts - one half was processed via the conventional density gradient method, and the other half was first mixed with HES, allowed to sediment, and then the supernatant was passed through a CIF-based microfluidic device (designed for high-precision separation of lymphocytes from smaller cells, and ~15-fold reduction of the sample volume) at a flow rate of 0.5 mL/min. For both separation methods, product yield and purity were quantified by performing CBC with a 5-part differential on all samples. Flow cytometry was used to measure viability of isolated lymphocytes, and activation level of residual platelets via P-selectin and phosphatidylserine (PS) exposure. Separated lymphocytes were cultured for 4 days using standard proliferation techniques. Total number of viable cells was determined using Trypan Blue exclusion dye and manual counting with a hemocytometer.
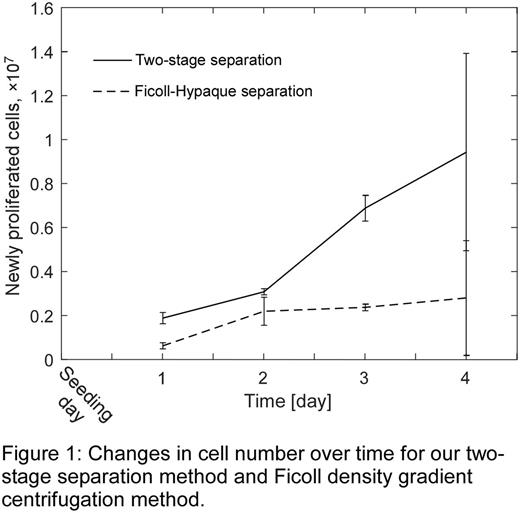
Results and Discussion: The optimized sedimentation protocol, which involved mixing WB with 10% HES (v/v) and waiting 30 min to complete, yielded the best combination of lymphocyte recovery (72.1±2.2%) and RBC depletion (99.48±0.07%) for the first stage of our separation approach. In the split-sample study, our two-stage method overall recovered 70.4±0.7% of lymphocytes from the initial WB sample, with 98.2±0.6% viability, while depleting 92.4±0.4% of platelets and 99.62±0.1% of RBCs. In comparison, the Ficoll density gradient centrifugation method removed 99.95±0.2% of RBCs and 94.2±0.1% of platelets, while recovering 74.5±2.40% of lymphocytes, with 98.3±0.7% viability. Lymphocytes produced by our method showed a significnatly higher prolifiration capacity than cells separated via density gradient centrifugation (Fig. 1). Markers of activation for residual platelets in the lymphocyte concentrate produced by our two-stage method were several fold lower than for Ficoll density gradient separation: 1.9±0.1% (our method) vs. 8.4±7.0% (Ficoll) for P-selectin, and 2.0±1.5% (our method) vs. 4.4±1.5% (Ficoll) for PS exposure. Importantly, lymphocyte isolation using our two-stage method was 3-fold faster than the Ficoll density graduate separation for the same amount of WB sample: <60 min (our method) vs. >180 min (Ficoll).
Conclusion: We developed an easy-to-use approach for extracting lymphocytes from peripheral blood with purity, viability and yield meeting the current standard for cellular therapies. Since our new approach is scalable, compatible with 'closed system' operation, produces lymphocytes with higher proliferation capacity, and is much faster than conventional density gradient separation, it could have a potentially transformative impact by accelerating research into new cellular therapies, and simplifying translation of these treatments into clinical practice.
Gifford: Halcyon Biomedical Incorporated: Employment, Equity Ownership. Shevkoplyas: Halcyon Biomedical Incorporated: Employment, Equity Ownership, Patents & Royalties: U.S. Patent Appl. 61/929,357, Research Funding; New Health Sciences, Inc.: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal